Iron Metabolism Part I: Sources, Transport, Testing
The Essentiality of Elemental iron
Iron is essential for healthy red blood cell hemoglobin levels. Red blood cell hemoglobin is essential for oxygen transport. Oxygen and glucose are absolutely essential for brain function. The topic of today’s review.
I think iron metabolism, assimilation and transport is highly misunderstood. And the subject of great controversy. Here is why. Hemoglobin is the essence of red blood cell function. And you can see in figure 1, iron is the essential core of hemoglobin. Healthy red blood cells transport oxygen. In plants, magnesium is absolutely essential for chlorophyll function.
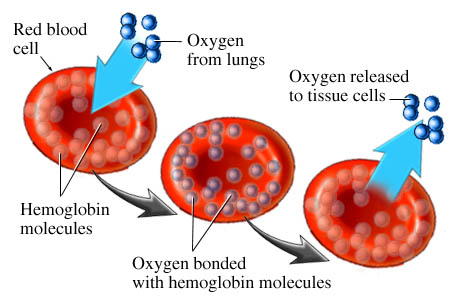
Red blood cells pass through the vasculature of your lungs. Oxygen is inhaled and absorbed by the circulating red blood cells. This is how oxygen is transported to the rest of your body. Your heart, brain and all other vital organs.
There is a widely held belief that iron supplementation is only essential for younger women especially and not as necessary in postmenopausal women. Similarly iron is believed to be less essential for men as we grow older. This is based on monthly menstrual cycles were iron is shed. In reality these differences in age are not that significantly different.
Sources of Iron
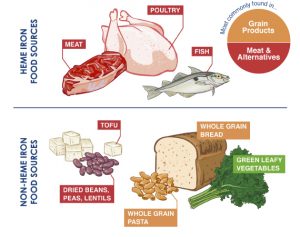
Iron is ingested in food sources or supplements. Beef, chicken liver, oysters, sardines, lentils, spinach.
But the plot thickens.
There is an important difference between heme-iron from red meats and seafoods and non-heme sources. Heme-iron is much more readily and efficiently absorbed. Non-heme sources are much less efficiently absorbed. All plant-based sources are non-heme iron. Additionally many plant based sources contain phytates, polyphenols, or soy that further inhibit efficient iron absorption.
This is why plant based diets so frequently lead to significant iron deficiency anemia. A source of heated controversy.
Elemental salts are also considered non-heme based. That would be ferrous sulfate, ferrous gluconate and ferrous fumarate salts. So simply looking a charts labeling the iron content of each nutrient is not enough. Chelated iron sources may lie in between.
Now let’s look at the continuum of iron deficiency to iron deficiency anemia. You can be iron deficient without suffering anemia. But eventually when iron levels are further depleted true iron deficiency anemia occurs.
Lab Testing — Hematology
Iron deficiency anemia is characterized by very small red blood cells – termed microcytosis. The MCV (the mean corpuscular volume) is a laboratory measure of your red blood cell size. An MCV < 88-90 infers iron deficiency.
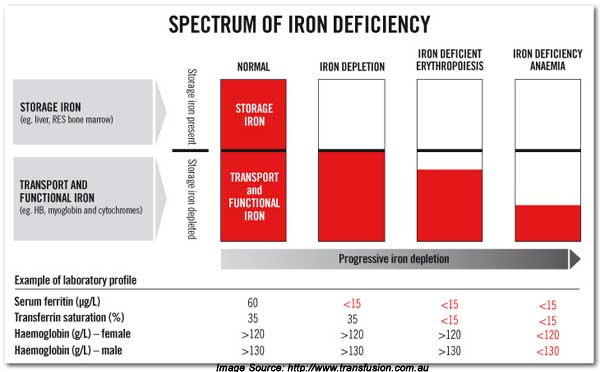
Figure 3
[The values in the above diagram are Australian values. Divide the hemoglobin values by 10 for American values. ]
Similarly an MCV > 100 (macrocytosis) raises the possibility of B12 or folate deficiency. .
These are not absolutes. They are general principles. There other conditions that raise the MCV. Alcohol is a major contributor to large (dysfunctional) red blood cells.
In Hematology we measure red blood cells and white blood cells. In the past a centrifuged (spun down) sample would contain a percentage of red blood cells with a top layer of serum. The percentage of sampled red blood cells is called the hematocrit (Hct). Today’s modern labs use flow cytometers so the hematocrit is inferred and not directly measured.
Hematologists have traditionally follow hemoglobin and not hematocrit. Which is probably more descriptive. We want to know how much hemoglobin is available. That is the oxygen carrier or transporter. In simple terms the hematocrit = 3 x hemoglobin.
Most labs use these ranges:
- Hct (women) 34-46% <34 is anemic optimal > 38
- Hct (men) 39-52% < 39 is anemic optimal > 44
- Hgb (women) 11-15 <11 is anemic optimal > 13
- Hgb (men) 12-17 < 12 is anemic optimal > 14
In practice, I use much tighter ranges. You are relatively anemic well before you reach these critical cutoff values. I have denote optimal values vs. strict lab cutoff values.
The third measurement is the actual RBC count. Your red blood cell mass.
-
Hematocrit
-
Hemoglobin
-
RBC count
-
MCV
Iron Metabolism and Transport
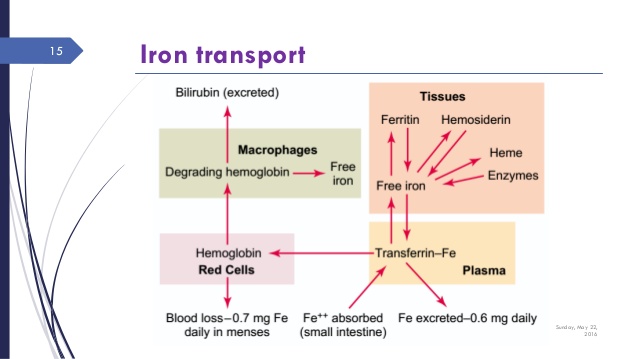
The movement and transport of iron is complex and subject to various controls. Figure 4 shows the movement of iron through these various “compartments.”
It is transported in the blood by attaching to transferrin. A large binding protein complex. It enters cellular storage through the control of ferroportin.
It is stored intracelluarly in the cellular cytosol as ferritin. The ferritin complex is a stable protective protein that neutralizes the reactive aspects of free ferrous (Fe³) iron.
Ferritin is the primary measure of stored iron. It is also a acute phase reactant. A biomarker of inflammation. This confounds ferritin as a simple measure of iron storage because it can also represent an inflammatory process.
Lab Testing — Iron BioMarkers
Now you can see from diagram 3 that measuring or following simple serum iron levels is inadequate. It is only a partial measure of iron sufficiency or deficiency. It is variable. To obtain a more accurate assessment and picture you must obtain:
-
Serum iron
-
Transferrin Iron saturation
-
Ferritin levels
-
TIBC (total iron binding capacity)
-
Serum transferrin receptor (sTfR) (added for subtle early signs of iron deficiency)
Ferritin is a measure of stored iron. Serum iron measures free floating iron. Because of insurance-based cost containment, ferritin is not routinely included in current laboratory testing. You definitely want ferritin levels.
Now the picture is even richer for the transport of iron. It becomes quite complex. Actually, far more complex than even I had ever imagined. I have often wondered about the relationship between ferritin (stored iron) and serum iron (free floating iron). What is the controlling factor or mechanism?
stay tuned for Part II — signals that control iron absorption